 Introduction
Introduction
Internal and external anti-infective coating of central venous catheters (CVCs) may reduce the rate of catheter colonization (CC) and blood stream infection (BSI) (1). Catheters with different types of anti-infective protection are available (1). New type of coated catheter (B.Braun) disposes of modified surface consisting of a high molecular weight polymer which is noncovalently linked to the polyurethane catheter material. The polymer consists of a methyl methacrylate backbone with four
different side chains. One of the side chains is derived from
a polymeric biguanide, chemically similar to the antiseptic polyhexanide. The matter is applied to the inner and outer catheter surface. Our objective was to evaluate the efficacy of coated catheter on the rate of CC and BSI in comparison with standard catheter in ICU settings.
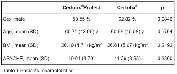 Methods
Methods
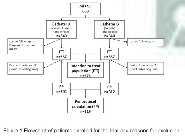
A prospective, randomized, controlled, double-blind clinical trial was performed on multidisciplinary ICUs of two university hospitals in the Czech Republic between 2005- 2010. A total of 680 patients were randomized to receive either coated CVC (Certofix®Protect, B.Braun Melsungen AG) or standard CVC (Certofix® B.Braun Melsungen AG). Catheters were inserted with full barrier precautions in the operating theatre or ICU. Catheter dressing, manipulation with sets and other care were delivered according hospital guidelines. Catheters were removed aseptically on physician indication and 5 cm long distal segment was sent for evaluation to the microbiology dept.
 Catheter colonization was defined as the growth of > 1000 colonyforming units using sonication method. Primary objectives were the difference of the incidence of both CC and BSI between groups. Secondary recorded data included clinical procedures and therapy, basic laboratory parameters (CRP, WBC), concomitant medication and invasive procedures, APACHE II, TISS scores and baseline patient data.
Catheter colonization was defined as the growth of > 1000 colonyforming units using sonication method. Primary objectives were the difference of the incidence of both CC and BSI between groups. Secondary recorded data included clinical procedures and therapy, basic laboratory parameters (CRP, WBC), concomitant medication and invasive procedures, APACHE II, TISS scores and baseline patient data.
 Results and Discussion
Results and Discussion
Total 674 catheters were evaluated among which 58 catheters were excluded due to short indwelling time < 3 days (an exclusion criterion). The two groups were similar with respect for the insertion site, place of insertion (ICU or surgical theatre), indwelling time, ICU stay and demographic
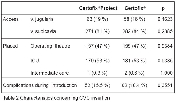
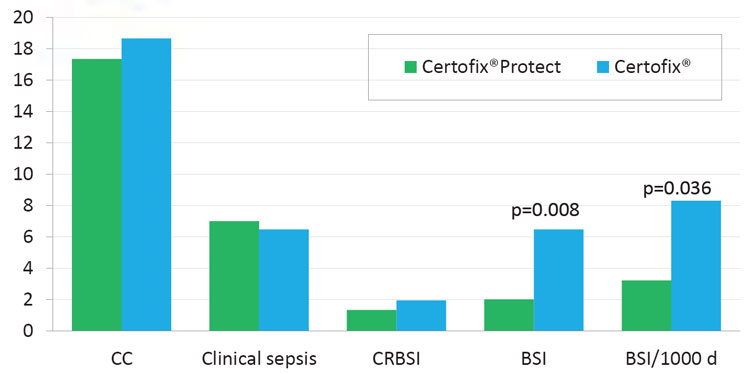
indices (Table 1 and Table 2). The coated CVC displayed similar incidence of CC as the standard CVCs (17.36 % vs. 18.67 %, p=0.747) as well as incidence of catheter-related BSI (1.33 % vs. 1.94 %, p=0.752). The rate of BSI was significantly lower in protected CVCs (2.00 % vs. 6.47 %, p=0.008), and the incidence of BSI/ 1000 catheter-days was lower in coated catheters (3.21 vs. 8.30, p=0.036) as well (Table 3 and Figure 2). There was no significant difference in ICU stay in days (8.55 vs. 8.82, p=0.743), catheter indwelling time (7.31 vs. 7.65, p=0.172) and other parameters (invasive procedures, complications).
 The difference between the groups was indentified in antibiotic therapy. More patients with standard CVC underwent antibiotic treatment due to infection in comparison to patients with coated CVC (88.16 % vs. 93.27%, p=0.036). This finding is probably related to higher incidence of BSI in control group. This is the first study comparing polyhexanide coated catheter to the standard CVC. The study was fully blinded and CC, BSI and CRBSI rates are comparable to other previously published studies (2,3). The finding that polyhexanide anti-infective coating reduces BSI rate and not the rate of CC could be probably more related to clinical data but remaining unexplained. One possible reason could be, that we didn´t took into account higher frequency of antibiotic administration at time of catheter removal in control group (4).
The difference between the groups was indentified in antibiotic therapy. More patients with standard CVC underwent antibiotic treatment due to infection in comparison to patients with coated CVC (88.16 % vs. 93.27%, p=0.036). This finding is probably related to higher incidence of BSI in control group. This is the first study comparing polyhexanide coated catheter to the standard CVC. The study was fully blinded and CC, BSI and CRBSI rates are comparable to other previously published studies (2,3). The finding that polyhexanide anti-infective coating reduces BSI rate and not the rate of CC could be probably more related to clinical data but remaining unexplained. One possible reason could be, that we didn´t took into account higher frequency of antibiotic administration at time of catheter removal in control group (4). Ivo Krikava1, Martin Kolar2, Barbora Garajova1, Tomas Balik2, Alena Sevcikova1, Jan Pachl2, Pavel Sevcik1,
Ivo Krikava1, Martin Kolar2, Barbora Garajova1, Tomas Balik2, Alena Sevcikova1, Jan Pachl2, Pavel Sevcik1,
Roman Trubac 3 1University Hospital Brno, KARIM, Brno, Czech Republic, 2University Hospital Kralovske Vinohrady, Dpt. of Anaesthesiology/CCM, Prague, Czech Republic
3B. Braun Medical s.r.o., Prague, Czech Republic


